Approximately 193000 atoms thick. Measure the mass of each of the squares of foil and record in the data table.
Heavy gauge aluminum foil refers to the aluminum foil paper with the thickness of 40 btm.
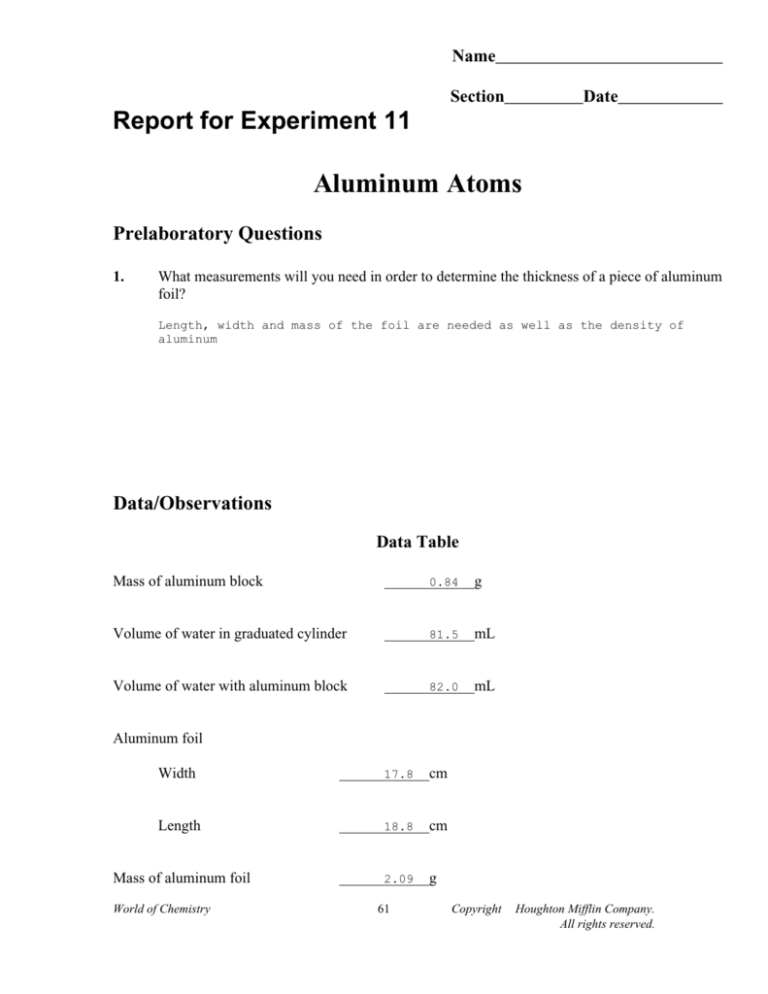
. 15710-3 2910-10 54138. The majority of Heavy Duty aluminum foil rolls are between 00008 and 0001 thick. Thus divided that number by area of foil to find that the foil is a few thousand atoms thick.
Texthow many atoms thick is standard aluminum foil And apologies if I am wrong The standard aluminum foil we use in the kitchen for baking spuds or lining trays has a thickness of 00165mm I. Approximately 193000 atoms thick. By dividing that by the surface area we found the thickness of the aluminum foil in cm.
How thick is a piece of aluminum foil. Divide its mass by its density. Use this formula and what you know about each foil square to.
If the area of foil is 1 sq. Convert pm to m. Convert m to cm.
By dividing the mass by a known density value we obtained the volume of aluminum foil in our sample. I think you mean to ask. How do you find the thickness of aluminum foil in an atom.
In the United States foils are commonly measured in thousandths of an inch or milsStandard household foil is typically. A sheet of aluminum foil has a total area of 1000 ft squared and a mass of 3636 grams. Record the volume in the data table.
Determine the volume of each foil square. Approximately 193000 atoms thick. A mole of aluminum weighs 27 grams The density of aluminum is 27 grams per cubic cm So one mole occupies a cube with volume of 10 cc.
Is there any way to determine the thickness of the aluminum foil in units of Al atoms. The extra three-thousandths of an inch makes a big difference for a variety of applications. We took squares of aluminum foil measured the surface area and mass.
Of gold 200approx So 200g of gold has 61023 atoms. Heavy gauge aluminum foil will withstand a lot of wear and tear. The density of Aluminum is 270 gcm3.
1 Al atom 286pm. We took squares of aluminum foil measured. Light gauge foil is the aluminum foil with the.
Extra-Heavy Duty Aluminum Foil Although very heavy-duty foil is tough to achieve certain manufacturers do manufacture it. Approximately 193000 atoms thick. Technically the thickness range of aluminum foil is between 0006mm 024 mils and 02 mm 8 mils.
In other words how many aluminum atoms thick is the aluminum foil. The thickness of aluminum foil is 6 microns or 0000234 inches. Consider the mass of foil 1210-17 gram.
Often informally called tin foil is aluminium prepared in thin metal leaves with a thickness less than 02 mm 79 mils. This is a fairly rough estimate from classroom data. Determine the number of aluminum atoms in your sample.
This is a fairly rough estimate from classroom data. Volume is determined by V l x w x h. Thank you for your participation.
Then the foil is 1000 atoms thick. You will need this to determine the volume. Most Aluminum foil rolls labeled Heavy Duty are between 0008 thick and 001 thick.
Answer 1 of 5. For a 100 cm2 piece of foil 10 cm by 10 cm the number of aluminum atoms will be about 1 x 1022. We took squares of aluminum foil measured the surface area and mass.
Calculate the thickness of the aluminum foil. The value was 286 10-3 cm The value converted to be 286105 angstroms. Because of its high level of puncture and tear resistance it is often deemed as the perfect material for more heavy duty applications.
For a variety of applications the extra three-thousandths of an inch makes a considerable difference. However the most common rolls used for household purposes are no thinner than 0016mm 063 mils and no thicker than 004mm 16 mils 1. By dividing that by the surface area we found the thickness of the aluminum foil in cm.
So the foil has 1000 atoms. Extra Heavy Duty Foil - A harder thickness to find but. Aluminum has a density of 270 gcm 3.
The value was 286 10. By dividing the mass by a known density value we obtained the volume of aluminum foil in our sample. How many Al atoms thick is the aluminum foil if it is 000157cm thick.
Divide aluminum thickness and Al atom thickness. How can you tell how many atoms of aluminum foil are thick. Density is mass divided by volume and the density of pure aluminumis well known to be 270 gcm3.
Question How many atoms thick is aluminum foil. By dividing the mass by a known density value we obtained the volume of aluminum foil in our sample. Thinner gauges down to 6 micrometres 024 mils are also commonly used.
Hope this answers your question. Aluminium foil or aluminum foil in North American English. Approximately 193000 atoms thick.

Lab 2 Thickness Of Aluminum Foil A E Chemistry Virtual Lab Youtube

Solved D Thickness Of Aluminum Foil Use The Photos Available On Canvas To Complete This Section Mass Of Foil Length Of Foil Width Of Foil L 839 15 55 M 12 Icm Calculation For
0 Comments